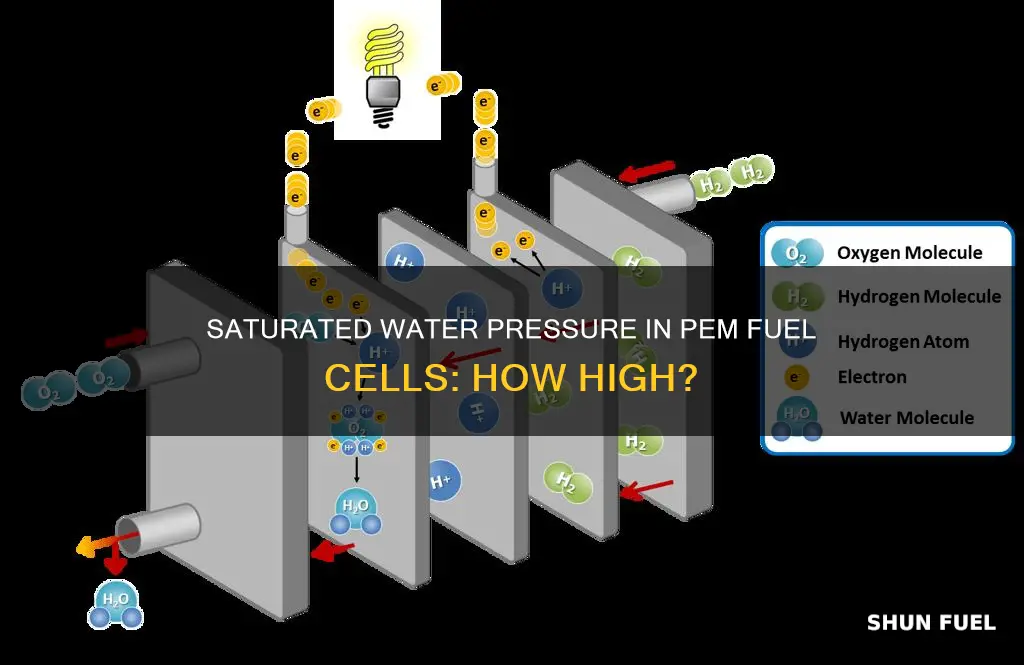
The pressure of saturated water in a PEM fuel cell is a crucial factor in its operation and performance. PEM fuel cells, which use hydrogen as fuel and emit water, are promising energy conversion systems that can replace internal combustion engines in automobiles due to their quick startup and low-emission nature. The operating pressure of a PEM fuel cell can be at ambient pressure or in a pressurized state, with fuel cell performance often improving with increased pressure. However, higher pressure can also lead to challenges in gas compression and storage, affecting the overall efficiency of the system.
One of the key challenges in PEM fuel cells is water management, which includes understanding and controlling water transport mechanisms such as electro-osmotic drag, back-diffusion, and convection. The water content in the membrane of the PEM fuel cell depends on factors such as operating temperature, pressure, and relative humidity. At the anode side, hydrogen oxidation occurs, and hydrogen atoms are converted into protons, dragging some water molecules with them as they move to the cathode side. This mechanism, known as electro-osmotic drag, results in a decrease in water concentration along the channel.
The operating temperature also plays a significant role in water management. At temperatures above 100 °C, the water byproduct becomes steam, reducing the criticality of water management in cell design. However, the upper limit of operation for PEM fuel cells is approximately 90 °C to prevent the membrane from drying out and causing a rapid performance drop. Therefore, the interplay between pressure, temperature, and relative humidity significantly influences the performance and stability of PEM fuel cells.
| Characteristics | Values |
|---|---|
| Operating Pressure | Ambient pressure or in a pressurized state |
| Operating Temperature | 50 to 100 °C |
| Flow Rates of Reactants | Equal to or greater than the rate at which reactants are consumed within the cell |
| Humidity of Reactants | 70% humidity achieved best performance in PEM fuel cell stacks |
What You'll Learn

Water management in PEM fuel cells
Water management is a critical aspect of proton exchange membrane (PEM) fuel cell operation and performance.
Fuel Pressure Regulator: Fixing a Faulty Part
You may want to see also

Operating temperature
At higher operating temperatures, PEM fuel cells exhibit You may want to see also The operating pressure of a PEM fuel cell also affects water management within the cell. As pressure increases, the saturation pressure of water vapour does not change for a constant operating temperature, but the mole fraction of an oxidant such as oxygen will increase. This can impact the water content within the cell, which is critical to its performance. The pressure difference between the inlet and outlet of the reactant gases in a PEM fuel cell is called the pressure drop, and it is related to the amount of water in the channels of the fuel cell. A higher pressure drop can indicate a higher water content, and this can be used to detect water faults within the cell. At high loads, a PEM fuel cell system is more efficient at higher air pressures and lower mass flow rates. Conversely, at low loads, the system efficiency is higher at lower air pressures and higher mass flow rates. The pressure within a PEM fuel cell also affects the velocity of the gases and liquids within the cell. For example, the velocity at the membrane layer is considerably lower than in the gas diffusion and reactant layers due to the smaller permeability coefficient of the membrane. Overall, the operating pressure of a PEM fuel cell has a significant impact on its performance and must be carefully considered and analysed to optimise the efficiency of the fuel cell system. You may want to see also The flow rate of reactants in a PEM fuel cell is a critical parameter influencing the overall performance of the fuel cell. It refers to the rate at which the reactants, such as hydrogen and oxygen, are introduced into the fuel cell system. Here are some key considerations regarding the flow rates of reactants: You may want to see also The humidity of reactants is a critical parameter in the operation of proton exchange membrane (PEM) fuel cells. PEM fuel cells require the proton exchange membranes to remain hydrated for optimal performance. If the membranes are not fully humidified, the conductivity decreases, resulting in increased energy consumption during proton transportation. Inadequate humidification can cause the membrane to cease functioning as a proton transporter. While the hydrogen and oxygen (or air) reactants produce water during the electrochemical reaction, the challenge lies in managing the water effectively to prevent flooding of the catalyst layer. This is where the Gas Diffusion Layer (GDL) plays a crucial role. GDL materials are designed with hydrophobic coatings and specific permeability properties to facilitate water removal from the catalyst layer while it is still in vapour form, preventing the formation of droplets that could block the catalyst. The humidity of reactants directly impacts the performance of PEM fuel cells. Research has shown that humidifying the reactant gases improves cell performance, particularly when the cathode gas is humidified. Higher inlet gas humidification levels and temperatures positively influence cell performance, with the membrane conductivity increasing as the water content rises. However, it is important to strike a balance as further increasing the water content can result in flooding, hindering reactant transport to the reaction zones. The optimal humidity conditions depend on various factors, including the flow field design and operating temperature. For instance, the influence of inlet relative humidity at the anode and cathode can be highly asymmetrical due to the different mechanisms involved. At the anode, humidification primarily affects membrane conductivity, while at the cathode, it influences liquid water saturation due to flooding. Therefore, the relative humidity of the reactants must be carefully controlled to ensure efficient PEM fuel cell operation. You may want to see also A proton-exchange membrane fuel cell (PEMFC), also known as a polymer electrolyte membrane (PEM) fuel cell, is a type of fuel cell with lower temperature/pressure ranges (50 to 100 °C) and a special proton-conducting polymer electrolyte membrane. A PEM fuel cell transforms the chemical energy liberated during the electrochemical reaction of hydrogen and oxygen into electrical energy. Hydrogen is delivered to the anode side of the membrane electrode assembly (MEA), where it is catalytically split into protons and electrons. The protons permeate through the polymer electrolyte membrane to the cathode side, while the electrons travel along an external load circuit to the cathode side of the MEA, creating a current. Meanwhile, oxygen is delivered to the cathode side of the MEA, where it reacts with the protons and electrons to form water molecules. Operating temperatures above 100 °C are desired so that the water byproduct becomes steam and water management becomes less critical in cell design. The upper limit of operation for PEMFCs is approximately 90 °C because water evaporates from the membrane at higher temperatures, drying it out and causing performance to drop. Fuel cell performance often improves with increased pressure. However, higher pressure can also make the system less efficient due to the need for gas compression and storage. Pressurization of the fuel also changes the water management in each cell.Fuel Injectors: Relieving Pressure for Better Performance?

Operating pressure
Fuel Pressure Fundamentals for Ecotec Engines

Flow rates of reactants
Removing Mustang Fuel Filter: Easy Steps to Follow

Humidity of reactants
Fuel Rail Pressure: Cummins' Common Cause and Fixes
Frequently asked questions







