The world is in a constant search for sustainable and clean energy sources to power our vehicles and industries. One promising alternative to gasoline is hydrogen, a versatile and abundant element that has the potential to revolutionize the way we fuel our transportation. Hydrogen fuel cells, which convert chemical energy into electricity, offer a cleaner and more efficient alternative to traditional combustion engines. With its zero-emission profile and the ability to be produced from renewable sources, hydrogen presents a compelling case for its use as a fuel. This paragraph introduces the topic of hydrogen's potential to replace gasoline, exploring the benefits, challenges, and possibilities of this emerging technology.
| Characteristics | Values |
|---|---|
| Energy Density | Hydrogen has a much lower energy density compared to gasoline, meaning it requires more volume to store the same amount of energy. |
| Storage and Infrastructure | Developing an infrastructure for hydrogen storage and distribution is a significant challenge. High-pressure tanks or cryogenic storage are currently used, but this requires specialized equipment and infrastructure. |
| Production Methods | Hydrogen can be produced through various methods, including electrolysis of water, steam reforming of natural gas, and biomass gasification. Each method has its own environmental impact and energy requirements. |
| Environmental Impact | Hydrogen itself is a clean fuel, producing only water vapor when burned. However, the production process can vary in its environmental footprint, depending on the energy source used. |
| Cost | The cost of hydrogen production and distribution is currently higher compared to gasoline. Scaling up production and improving technologies can potentially reduce costs over time. |
| Vehicle Range | Hydrogen fuel cell vehicles can offer a similar range to gasoline vehicles, but refueling stations are less common, which can be a limitation. |
| Efficiency | Hydrogen fuel cells are generally more efficient than internal combustion engines, converting a higher percentage of energy to power. |
| Availability | Hydrogen is abundant and can be produced from various sources, but the infrastructure to support its widespread use is still developing. |
| Safety | Hydrogen is a highly flammable gas, and proper safety measures are necessary during storage, transportation, and use. |
| Infrastructure Compatibility | Adapting existing fuel infrastructure to accommodate hydrogen would require significant investment and changes. |
What You'll Learn
- Production Methods: Electrolysis, steam methane reforming, and biomass gasification for hydrogen generation
- Storage and Transportation: High-pressure tanks, liquid hydrogen, and metal hydrides for efficient storage and transport
- Vehicle Integration: Fuel cells, internal combustion engines, and hydrogen-powered electric motors for vehicle propulsion
- Infrastructure Development: Hydrogen refueling stations, pipelines, and distribution networks for widespread adoption
- Environmental Impact: Reduced carbon emissions, air quality improvements, and sustainable energy sources compared to gasoline

Production Methods: Electrolysis, steam methane reforming, and biomass gasification for hydrogen generation
The production of hydrogen for use as a fuel is an area of active research and development, with several methods being explored to generate this clean energy source. Here, we delve into three key production methods: electrolysis, steam methane reforming, and biomass gasification.
Electrolysis: This process involves passing an electric current through water to split it into hydrogen and oxygen. The reaction is as follows: 2H₂O → 2H₂ + O₂. Electrolysis is a clean and renewable method of hydrogen production, especially when powered by renewable energy sources like solar or wind. The hydrogen produced can be stored and used directly in fuel cells or compressed for later use. However, the efficiency of electrolysis can be affected by factors such as the temperature and pressure of the water, as well as the type of electrolyte used.
Steam Methane Reforming (SMR): This is a widely used industrial process for hydrogen production, particularly in the oil and gas industry. SMR involves reacting methane (CH₄) with steam (H₂O) at high temperatures (around 700-1100°C) to produce hydrogen and carbon monoxide (CO). The chemical equation is: CH₄ + 2H₂O → CO + 4H₂. The hydrogen generated can then be separated and used as a fuel. While SMR is an efficient method, it is not considered renewable due to its reliance on fossil fuels like methane. However, efforts are being made to couple SMR with carbon capture and storage technologies to reduce its environmental impact.
Biomass Gasification: This method involves heating biomass (organic matter) in the absence of oxygen to produce a gas known as syngas (synthesis gas). The process can be represented by the equation: C₃H₈ + 3O₂ → 3CO₂ + 4H₂O + energy. Syngas is a mixture of carbon monoxide (CO) and hydrogen (H₂), which can be further processed to produce hydrogen. Biomass gasification offers a renewable and sustainable way to generate hydrogen, especially when using agricultural waste, wood chips, or dedicated energy crops. The gas produced can be used directly in fuel cells or converted to hydrogen through a process called 'shift reaction'. This method is particularly promising for rural or remote areas where hydrogen infrastructure may not be readily available.
Each of these production methods has its advantages and challenges, and the choice of method depends on factors such as availability of feedstock, energy sources, and infrastructure. Electrolysis and biomass gasification offer renewable and sustainable options, while SMR is more established but relies on fossil fuels. The development of efficient and cost-effective hydrogen production methods is crucial for the successful transition from gasoline to hydrogen as a fuel source.
Replacing Fuel Pump in Passat: Step-by-Step Guide
You may want to see also

Storage and Transportation: High-pressure tanks, liquid hydrogen, and metal hydrides for efficient storage and transport
The storage and transportation of hydrogen present unique challenges that need to be addressed for its widespread adoption as a fuel. One of the primary methods of storing hydrogen is through high-pressure tanks, which are commonly used in the automotive industry. These tanks are designed to hold hydrogen at pressures ranging from 350 to 700 bars (5,000 to 10,000 psi), allowing for a reasonable driving range in fuel cell vehicles. The high-pressure storage system is compact and lightweight, making it suitable for vehicles where space and weight are critical factors. However, the high pressure required for storage poses safety concerns, and specialized materials and designs are necessary to ensure the integrity of the tanks.
Another approach to hydrogen storage is through the use of liquid hydrogen. This method involves cooling hydrogen to extremely low temperatures, typically below -253°C (-423°F), until it becomes a liquid. Liquid hydrogen has a much higher energy density compared to gaseous hydrogen at standard conditions, making it more efficient for energy storage. It can be stored in specialized tanks and transported over long distances. However, the process of liquefaction and regasification requires significant energy input, and the infrastructure for handling and storing liquid hydrogen is more complex and expensive.
Metal hydrides offer a promising alternative for hydrogen storage and transportation. These compounds can absorb and release hydrogen gas through chemical reactions, providing a safe and efficient storage method. Sodium alanate (NaAlH4) is a well-known metal hydride that can store hydrogen at relatively high densities. Metal hydride storage systems can be designed to release hydrogen as needed, making them suitable for various applications, including stationary power generation and transportation. The development of metal hydride-based storage systems has been an active area of research, aiming to improve their reversibility, hydrogen storage capacity, and cycle life.
In addition to storage, the transportation of hydrogen also requires careful consideration. High-pressure hydrogen gas can be transported in specialized pipelines, similar to natural gas distribution systems. However, the infrastructure for hydrogen transportation needs to be carefully designed to handle the high pressure and potential hazards associated with hydrogen. Alternatively, liquid hydrogen can be transported in insulated tanks, ensuring that it remains in a liquid state during transit. The development of efficient and safe transportation methods is crucial for establishing a hydrogen supply chain, connecting production sites to end-users.
The challenges of hydrogen storage and transportation are significant but not insurmountable. High-pressure tanks provide a practical solution for automotive applications, while liquid hydrogen and metal hydrides offer alternative storage options with their own advantages. The development of efficient and safe storage and transportation methods is essential for the successful integration of hydrogen into the energy landscape, potentially replacing gasoline as a primary fuel source. Ongoing research and investment in these areas will drive the advancement of hydrogen technology and its widespread adoption.
Replacing the Fuel Door Spring: A Step-by-Step Guide for 2007 Tahoe Owners
You may want to see also

Vehicle Integration: Fuel cells, internal combustion engines, and hydrogen-powered electric motors for vehicle propulsion
The transition from gasoline to hydrogen as a primary vehicle fuel is an intriguing prospect, offering a cleaner and potentially more sustainable alternative. This shift involves integrating various technologies, including fuel cells, internal combustion engines, and hydrogen-powered electric motors, to ensure efficient and effective vehicle propulsion.
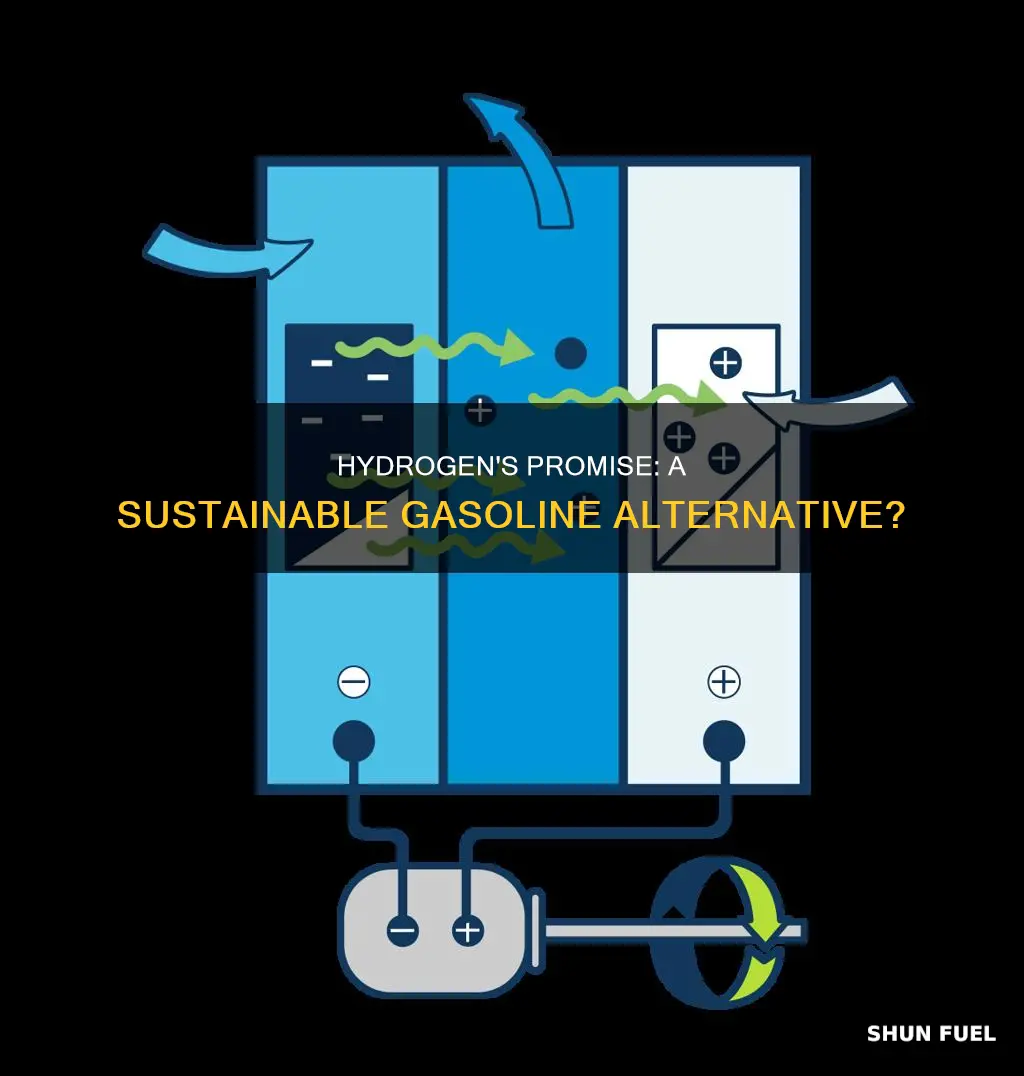
Fuel Cells: These devices are at the heart of hydrogen-powered vehicles. A fuel cell generates electricity through an electrochemical reaction between hydrogen and oxygen, producing water as a byproduct. This process is highly efficient, converting most of the energy in hydrogen to electricity, which then powers the vehicle's electric motor. Fuel cells are known for their quiet operation, zero tailpipe emissions, and rapid refueling, making them an attractive option for electric vehicles (EVs). Integrating fuel cells into vehicles requires careful design to manage the high-pressure hydrogen storage and ensure a safe and efficient energy supply.
Internal Combustion Engines (ICEs): While less common in modern hydrogen vehicles, ICEs can still play a role in the transition. Hydrogen ICEs offer a familiar and established technology, as many vehicles already use gasoline ICEs. Adapting these engines to run on hydrogen involves modifying the combustion chamber and fuel injection system. Hydrogen ICEs can provide a range of benefits, including reduced emissions and improved fuel efficiency compared to gasoline engines. However, the challenge lies in optimizing the combustion process to ensure efficient and controlled burning of hydrogen, which is a more difficult task than with gasoline.
Hydrogen-Powered Electric Motors: This approach combines the advantages of both fuel cells and traditional electric motors. In this system, hydrogen is used to generate electricity through a fuel cell, which then powers an electric motor. This setup offers the benefits of zero-emission driving, rapid refueling, and potentially higher efficiency compared to direct hydrogen combustion in an ICE. The electric motor's role is to convert the electrical energy into mechanical power, driving the vehicle's wheels. This integration allows for a more flexible and adaptable propulsion system, as the electric motor can be used in conjunction with or independently of the fuel cell, depending on the vehicle's needs.
Integrating these technologies into vehicles requires a comprehensive understanding of hydrogen's unique properties and challenges. Hydrogen has a lower energy density than gasoline, meaning more fuel is needed to achieve the same range. This issue is partially addressed by using high-pressure storage tanks or liquid hydrogen, but it remains a critical consideration in vehicle design. Additionally, the infrastructure for hydrogen refueling stations is still developing, and ensuring a widespread and accessible network is essential for the widespread adoption of hydrogen-powered vehicles.
In summary, replacing gasoline with hydrogen as a vehicle fuel involves a complex integration of fuel cell technology, internal combustion engines, and hydrogen-powered electric motors. Each component offers unique advantages, and their successful implementation will depend on addressing the challenges of hydrogen storage, refueling infrastructure, and engine optimization. As research and development continue, the potential for hydrogen to become a viable and sustainable transportation fuel becomes increasingly realistic.
Fuel Lines: Replacing Them Before They Become a Problem
You may want to see also

Infrastructure Development: Hydrogen refueling stations, pipelines, and distribution networks for widespread adoption
The transition from gasoline to hydrogen as a primary fuel source requires a comprehensive infrastructure development strategy, focusing on hydrogen refueling stations, pipelines, and distribution networks. These elements are crucial for ensuring the widespread adoption of hydrogen fuel cell vehicles and addressing the challenges associated with its storage, transportation, and delivery.
Hydrogen Refueling Stations:
The establishment of a robust network of hydrogen refueling stations is essential for the practical use of hydrogen fuel cell vehicles. These stations serve as the 'gas stations' for hydrogen, allowing drivers to refuel their vehicles conveniently. The design and placement of these stations should consider factors such as vehicle density, population distribution, and travel patterns. For instance, in urban areas with high vehicle concentrations, a higher density of refueling stations might be required to support the daily needs of hydrogen-powered fleets. Each station should be equipped with specialized hydrogen dispensing equipment, ensuring efficient and safe refueling processes. The infrastructure should also include safety measures to prevent hydrogen leaks and mitigate potential hazards, adhering to strict industry standards and regulations.
Pipelines and Distribution Networks:
Transporting hydrogen over long distances efficiently and safely is a critical aspect of infrastructure development. Hydrogen pipelines, similar to natural gas pipelines, can be utilized to move hydrogen from production facilities to refueling stations. These pipelines need to be designed with advanced materials and technologies to withstand the unique properties of hydrogen, including its low boiling point and high compressibility. The distribution network should also include compressed gas storage facilities and cryogenic tanks for hydrogen, ensuring a stable supply and addressing the challenges of hydrogen's low-temperature storage requirements. Additionally, the development of hydrogen-specific transportation routes and logistics systems is necessary to manage the delicate nature of hydrogen shipments.
Widespread Adoption and Integration:
The success of hydrogen infrastructure relies on its seamless integration into existing energy systems and transportation networks. This involves collaboration between various stakeholders, including energy companies, transportation authorities, and government bodies. Widespread adoption can be facilitated by offering incentives and subsidies to encourage the construction of refueling stations and the development of hydrogen-ready vehicles. Furthermore, the integration of hydrogen production and distribution with other energy sources, such as renewable energy systems, can create a sustainable and environmentally friendly energy ecosystem.
In summary, the infrastructure development for hydrogen fuel's widespread adoption involves a multi-faceted approach. It encompasses the strategic placement of hydrogen refueling stations, the construction of specialized pipelines and distribution networks, and the integration of hydrogen with existing energy systems. By addressing these infrastructure challenges, we can pave the way for a successful transition from gasoline to hydrogen, contributing to a cleaner and more sustainable energy future.
Fuel Filter Replacement: A Step-by-Step Guide for 2003 Mitsubishi Outlander Owners
You may want to see also

Environmental Impact: Reduced carbon emissions, air quality improvements, and sustainable energy sources compared to gasoline
The potential of hydrogen as a clean energy source has sparked significant interest in its ability to replace gasoline as a fuel, especially in the context of environmental sustainability. One of the most compelling advantages of hydrogen is its role in reducing carbon emissions. When hydrogen is used as a fuel, it undergoes a chemical reaction with oxygen, producing water as the primary byproduct. This process is in stark contrast to the combustion of gasoline, which releases a significant amount of carbon dioxide (CO2) and other harmful pollutants into the atmosphere. By adopting hydrogen as a fuel, we can significantly lower the carbon footprint of the transportation sector, which is a major contributor to global greenhouse gas emissions.
The environmental benefits of hydrogen extend beyond carbon emissions. The widespread use of hydrogen fuel can lead to substantial improvements in air quality. Gasoline combustion releases not only CO2 but also a range of toxic pollutants, including nitrogen oxides (NOx), volatile organic compounds (VOCs), and particulate matter. These pollutants have detrimental effects on human health, causing respiratory issues and contributing to the formation of smog. Hydrogen, when used as a fuel, produces no such harmful emissions, making it an attractive alternative for reducing air pollution in urban areas.
Furthermore, hydrogen's potential as a sustainable energy source is a key factor in its consideration as a replacement for gasoline. Unlike gasoline, which is derived from finite fossil fuels, hydrogen can be produced from renewable sources such as wind, solar, and hydroelectric power. This renewable production process ensures a more sustainable and environmentally friendly supply of energy. For instance, electrolysis of water using renewable electricity can generate hydrogen with a minimal carbon footprint, making it a truly green energy carrier.
The environmental impact of hydrogen as a fuel also includes the potential for a more efficient and sustainable energy infrastructure. Hydrogen can be stored and transported in various forms, including compressed gas, liquid, and as a fuel cell. This versatility allows for the development of a flexible energy system that can integrate with existing infrastructure while also supporting the growth of renewable energy sources. Additionally, the use of hydrogen fuel cells in vehicles and other applications can provide a more efficient and cleaner way of powering transportation, further reducing the environmental impact of the mobility sector.
In summary, the environmental benefits of using hydrogen to replace gasoline are significant. Hydrogen fuel offers a pathway to reduce carbon emissions, improve air quality, and transition towards more sustainable energy sources. As the world seeks to mitigate the impacts of climate change and promote cleaner energy alternatives, hydrogen's potential as a clean and renewable fuel is an exciting prospect that warrants further exploration and investment.
Toyota Camry Fuel Filter: A Step-by-Step Guide to Replacement
You may want to see also
Frequently asked questions
Hydrogen has the potential to be a clean and sustainable alternative to gasoline for transportation. It can be used as a fuel for vehicles, particularly in fuel cell electric vehicles (FCEVs), which produce electricity through an electrochemical reaction between hydrogen and oxygen, emitting only water vapor and warm air. This makes hydrogen an attractive option for reducing greenhouse gas emissions and air pollution.
Hydrogen offers several advantages as a fuel source. Firstly, it has a high energy density, allowing for efficient energy storage and longer driving ranges compared to battery-electric vehicles. Secondly, hydrogen can be produced from renewable sources like wind and solar power, making it a sustainable and environmentally friendly option. Additionally, hydrogen refueling stations can be quickly established, similar to gasoline stations, providing convenience for drivers.
While hydrogen has great potential, there are some challenges to its widespread adoption. One major challenge is the infrastructure required for hydrogen production, storage, and distribution. Building a comprehensive network of hydrogen refueling stations is essential but currently lacking in many regions. Another consideration is the energy efficiency of the entire process, from hydrogen production to vehicle usage, which needs to be optimized to make it cost-effective and competitive with gasoline.







