Crude oil is a fossil fuel that is pumped or mined from underground reservoirs. It is a combination of hydrocarbons that have been exposed to heat and compression over millions of years. To be used as fuel, it must be refined to remove impurities and separated into different products. This is done through a process called fractional distillation, where the oil is heated at different temperatures to separate the different substances. This process breaks down the molecular chains of carbon in the oil, allowing the lighter gases to be collected for fuel. The oil is then converted by 'cracking' heavy hydrocarbon molecules into lighter, smaller molecules. Finally, the oil is treated to remove impurities such as sulfur and nitrogen, which can cause air pollution.
| Characteristics | Values |
|---|---|
| What fuel oil is changed into | Gasoline, diesel fuel, and jet fuel |
| What is fuel oil | A fossil fuel, a mixture of hydrocarbons that formed from the remains of animals and plants |
| How is fuel oil changed into gasoline | Through a refining process that includes distilling, converting, and treating |
| What is distilling | A process where oil is heated in large, tall towers to break down the molecular chains of carbon in the oil, making it separate into layers |
| What is converting | A process where heavy hydrocarbon molecules are 'cracked' into lighter, smaller molecules through a reaction between the oil and hydrogen under high pressure and heat |
| What is treating | A process where impurities such as sulfur and nitrogen are removed from the petroleum |
What You'll Learn

Crude oil is heated in a distillation column
Crude oil is a combination of hydrocarbons found underground. It comes in several variations, such as light, medium, heavy, and extra-crude, each with different viscosities, densities, and sulfur content. In its natural state, it has very little value, so the first step in processing it into gasoline is to separate its components. This is done through a process called fractional distillation, which separates the various components of the crude oil by their size, weight, and boiling point.
Crude oil is first heated and then put into a distillation column, also known as a still, where different products boil off and are recovered at different temperatures. The distillation column contains several trays, each designated for a different kind of oil. The vapors from the heated crude oil rise up into the column, and the trays capture the vapors as they separate according to their individual boiling points. The substance with the lowest boiling point will condense at the highest point in the column, while the substance with the highest boiling point will condense lower down.
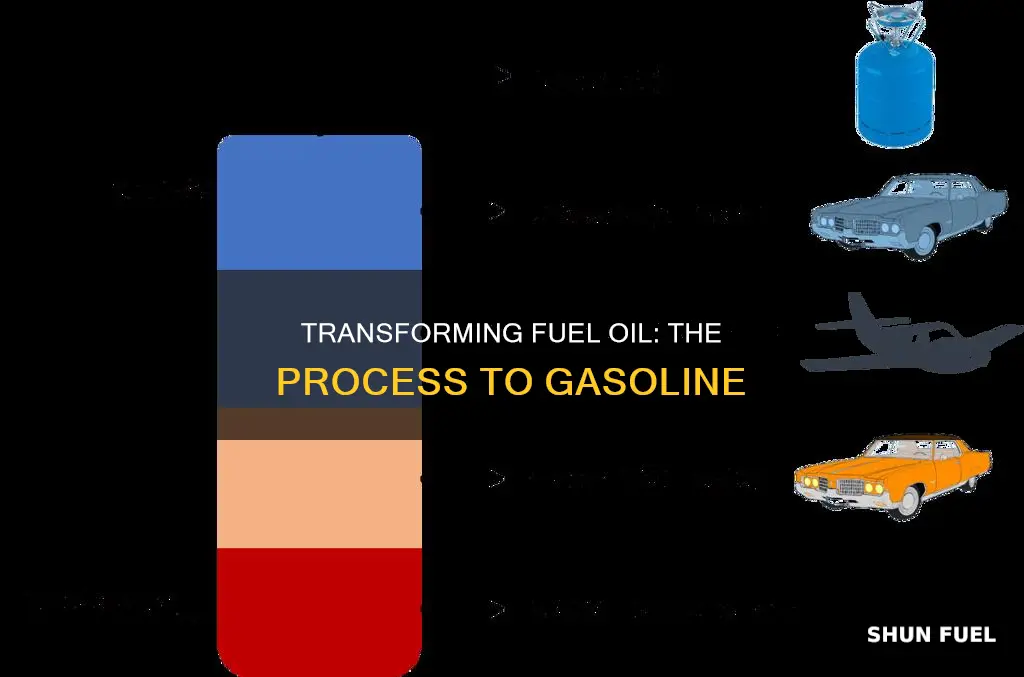
Lighter products, such as butane and other liquid petroleum gases (LPG), gasoline blending components, and naphtha, are recovered at the lowest temperatures. These light fractions vaporize and rise to the top of the distillation tower, where they condense back into liquids. Mid-range products, including jet fuel, kerosene, and distillates (such as heating oil and diesel fuel), are found in the middle of the tower. The lightest fractions, including gasoline, are then sent through pipes to different areas for further processing.
After distillation, the heavy, lower-value distillation fractions can be processed further into lighter, higher-value products such as gasoline. This is done through a process called cracking, which breaks down large molecules into smaller ones. There are two types of cracking mechanisms: thermal cracking, which heats the oil to high temperatures, and catalytic cracking, which uses a catalyst to promote the breakdown of large molecules at lower temperatures. This process ensures that the gasoline has desirable properties, such as a low sulfur content and a high octane rating.
Fuel Pump and Relay: When to Change Them Both
You may want to see also

Different substances are separated based on their boiling points
The process of converting fuel oil into gasoline involves several steps, including distillation, conversion, and treatment. Distillation is the first critical step in this transformation.
During distillation, the crude oil is heated in large, tall towers, causing it to vaporize. The extremely high temperatures break down the molecular chains of carbon in the oil, leading to separation into layers based on their boiling points. This separation is possible because each molecule in crude oil boils at a different temperature. The heavy elements, such as sludge, sink to the bottom, while lighter gases like propane float to the top. The oil that will eventually be converted into gasoline and jet fuel remains suspended in the middle.
The separated hydrocarbons then cool and condense at various temperatures and levels within the distillation tower. The cooling process is essential for the hydrocarbons to transition from a gaseous state back to a liquid state. As this occurs, the heavy asphalt and tar hydrocarbons condense at the bottom of the tower, while the light natural gas hydrocarbons collect at the top. This cooling and condensation process further refines the separation of substances based on their boiling points.
After distillation, the substances undergo a process called "cracking." During cracking, larger hydrocarbon molecules are broken down into smaller ones, which are used to produce gasoline. This step ensures that the resulting gasoline has desirable properties, such as a low sulfur content and a high octane rating. There are two methods of cracking: catalytic cracking and thermal cracking. Catalytic cracking utilizes a catalyst to accelerate the breakdown of larger molecules, while thermal cracking involves heating the oil to extremely high temperatures, typically in the range of 900°F to 1,000°F.
In summary, the conversion of fuel oil into gasoline involves a series of steps, with distillation being the key process that separates substances based on their boiling points. This separation allows for the production of gasoline and other valuable petroleum products.
Fixing Fuel Pressure: Replacing the Regulator
You may want to see also

Large hydrocarbon molecules are 'cracked' into smaller molecules
Large hydrocarbon molecules are cracked into smaller molecules through a process known as "cracking". This process is an essential part of refining crude oil into gasoline and other useful products. Cracking involves breaking down complex organic molecules, such as long-chain hydrocarbons, into simpler molecules, such as light hydrocarbons, by breaking the carbon-carbon bonds in the precursors.
There are several methods of cracking, but the two main ones are catalytic cracking and steam cracking. Catalytic cracking uses a catalyst, typically a zeolite, to speed up the breakdown of larger molecules. The long-chain hydrocarbon is turned into a gas, which then passes over a hot catalyst at a temperature of about 550°C. The long-chain hydrocarbon splits into shorter-chain hydrocarbons as it passes over the catalyst.
Steam cracking, on the other hand, simply involves heat and steam. The long-chain hydrocarbon is turned into a gas and mixed with steam. At very high temperatures, typically over 850°C, and under pressure, the long-chain hydrocarbon will split into shorter-chain hydrocarbons and many small alkenes. The alkenes are then separated from the alkanes by fractional distillation.
The choice between catalytic and steam cracking depends on the desired products and the nature of the feedstock. Catalytic cracking is generally preferred as it produces higher yields of useful products and requires less energy. However, steam cracking can be useful for producing certain chemicals, such as ethylene and propylene, which are valuable feedstocks for the petrochemical industry.
The process of cracking is important for several reasons. Firstly, it helps match the supply of fractions produced by fractional distillation with the demand for them. Secondly, it produces alkenes, which are useful as feedstock for the petrochemical industry. Finally, smaller hydrocarbons produced by cracking are more useful as fuels as they are less viscous and more flammable than larger hydrocarbons.
Replacing Fuel Filters: Step-by-Step Guide for Fass Systems
You may want to see also

Hydrogen and a catalyst are used to remove sulfur
Hydrogen and a catalyst are used in a process called
How to Change ECM Resistance for W Fuel Injectors
You may want to see also

Refineries blend hydrocarbons based on the end user's needs
The first step in the refining process is distillation. This includes heating crude oil at extreme temperatures to separate the different hydrocarbons. Oil refineries are typically large, with extensive piping running throughout, carrying streams of fluid between large chemical processing units, such as distillation columns.
After distillation, the next step in the refining process is typically cracking. This process breaks large molecules into smaller ones, transforming the materials in the distilled product into more desirable ones. There are two types of cracking mechanisms: thermal and catalytic. Thermal cracking involves heating the distilled product to high temperatures to break molecules, mainly producing gasoline and diesel. Catalytic cracking uses a catalyst to promote the breakdown of large molecules of carbon atoms into smaller molecules at high temperatures.
Following the cracking process, the different products are then blended to create the finished products that are sold to consumers. In addition to crude oil, refineries may also use other oils and liquids during this blending process, such as lease condensates, natural gas plant liquids, liquefied gases, and unfinished oils.
Suzuki Motorcycle Fuel Injection: Adjusting to Exhaust Changes
You may want to see also
Frequently asked questions
Fuel oil is a product of refined crude oil. It is a highly flammable, energy-dense liquid that evaporates quickly.
Crude oil is a mixture of chemicals that are separated through a process called fractional distillation. This involves heating the oil at different temperatures and collecting the different substances that boil at those temperatures. After distillation, the oil is refined to remove impurities. Then, it undergoes a process called "cracking", where heavy hydrocarbon molecules are broken down into lighter, smaller molecules. Finally, the hydrocarbons are blended together to create gasoline.
The first step is to heat the crude oil in a furnace until most of it vaporizes into a gas.
Distillation separates the liquids and vapours that result from heating the crude oil into different streams, or fractions, based on their boiling points. This allows for the production of products with distinct physical properties.
"Cracking" is the process of breaking down large hydrocarbon molecules into smaller ones. This is done through the use of heat, pressure, and catalysts.







