Hydrogen fuel cell cars are an innovative technology that offers a promising alternative to traditional internal combustion engines. One of the key questions surrounding these vehicles is whether they require oxygen to operate. Unlike conventional cars that burn gasoline or diesel, hydrogen fuel cell cars generate electricity through a chemical reaction between hydrogen and oxygen. This reaction produces water as a byproduct, making hydrogen fuel cell cars an environmentally friendly option. However, the question of whether oxygen is necessary for their operation is intriguing. In this paragraph, we will explore the relationship between hydrogen fuel cell cars and oxygen, shedding light on the unique process that powers these vehicles and addressing the common misconception about their oxygen requirements.
| Characteristics | Values |
|---|---|
| Oxygen Requirement | Hydrogen fuel cells do not require oxygen to operate. They produce electricity through a chemical reaction between hydrogen and oxygen, but the oxygen is not needed for the process itself. |
| Combustion | Unlike traditional internal combustion engines, hydrogen fuel cells do not burn hydrogen. Instead, they use a catalyst to split hydrogen molecules into protons and electrons, generating electricity. |
| Efficiency | Hydrogen fuel cells are highly efficient, converting a large portion of the chemical energy in hydrogen to electrical energy. This efficiency can lead to reduced energy consumption and lower emissions compared to conventional vehicles. |
| Environmental Impact | Hydrogen fuel cells produce only water and heat as byproducts, making them environmentally friendly. However, the production and storage of hydrogen can have environmental implications, especially if renewable energy sources are not used. |
| Energy Storage | Hydrogen can be stored as a gas, liquid, or in metal hydrides. This allows for various storage methods, including high-pressure tanks, cryogenic storage, and onboard storage systems. |
| Range | Hydrogen fuel cell vehicles can achieve a range comparable to or even exceeding that of battery electric vehicles, depending on the fuel cell system and storage capacity. |
| Refueling Time | Refueling a hydrogen fuel cell vehicle is generally faster than recharging a battery electric vehicle, typically taking a few minutes. |
| Infrastructure | The availability of hydrogen refueling stations is crucial for the widespread adoption of hydrogen fuel cell vehicles. Infrastructure development is an ongoing challenge. |
| Cost | The cost of hydrogen fuel cell vehicles and infrastructure has been a barrier to market penetration. However, costs are expected to decrease as technology advances and production scales up. |
| Safety | Hydrogen is a highly flammable gas, and safety measures are essential. These include pressure regulation, leak detection, and proper storage and handling procedures. |
What You'll Learn
- Hydrogen Fuel Cells: Hydrogen fuel cells convert hydrogen and oxygen into electricity, water, and heat
- Oxygen Requirement: Hydrogen fuel cells require oxygen for combustion, but not for the fuel cell reaction
- Air Intake: Cars with hydrogen fuel cells need air intake systems to supply oxygen for combustion
- Storage and Delivery: Oxygen storage and delivery systems are crucial for hydrogen fuel cell vehicles
- Environmental Impact: Hydrogen fuel cars produce water vapor and no harmful emissions, reducing air pollution

Hydrogen Fuel Cells: Hydrogen fuel cells convert hydrogen and oxygen into electricity, water, and heat
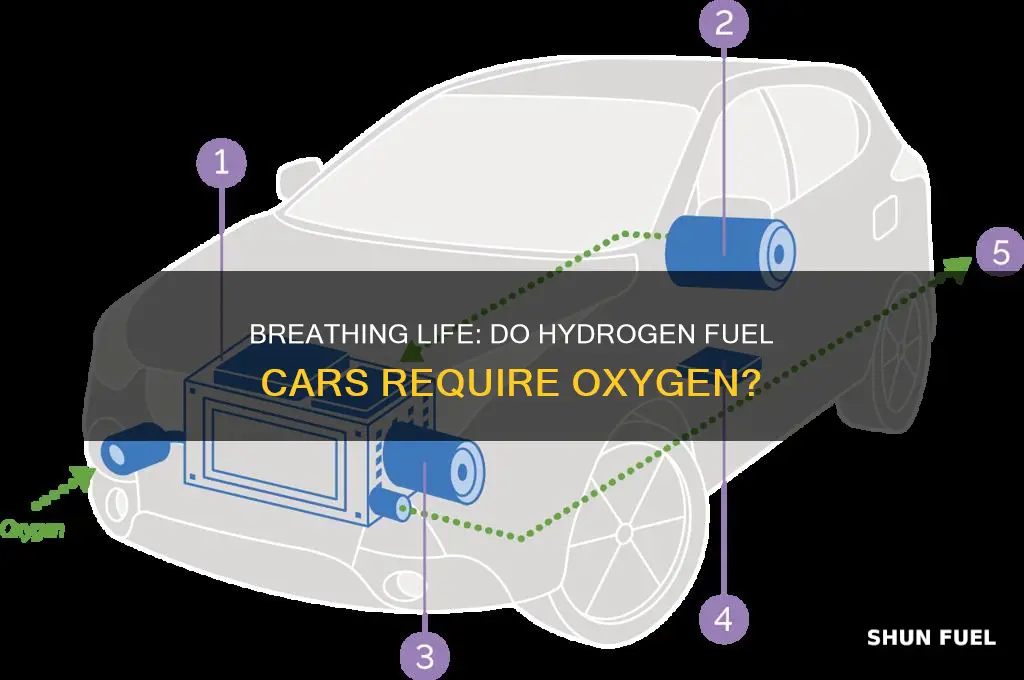
The concept of hydrogen fuel cells is an intriguing one, offering a potential solution to the world's growing energy demands and environmental concerns. These devices are designed to harness the power of hydrogen, a simple yet highly reactive element, and transform it into a sustainable source of energy. At the heart of this process lies the fundamental principle of electrochemical reactions, where hydrogen and oxygen, two of the most abundant elements in the universe, undergo a transformation to produce electricity, water, and heat.
In the intricate dance of a hydrogen fuel cell, hydrogen gas, typically in the form of compressed hydrogen, enters the cell and encounters a catalyst, often made of a precious metal like platinum. This catalyst facilitates a chemical reaction where hydrogen molecules split into protons and electrons. The electrons, eager to maintain a stable configuration, flow through an external circuit, creating an electric current. This current is the electricity we can harness for various applications. The protons, meanwhile, migrate through a special membrane, a critical component that selectively allows protons to pass while blocking electrons. This membrane is designed to maintain the separation of the electron flow, ensuring the efficiency of the process.
As the electrons traverse the circuit, they are guided to react with oxygen, the other essential reactant. This reaction occurs at the cell's cathode, where oxygen gas is fed in. Here, the electrons combine with oxygen to form water, a byproduct that is harmless and environmentally friendly. This process is the essence of the fuel cell's power generation, where the energy stored in hydrogen is converted into usable electricity, with water as the only emission.
The beauty of hydrogen fuel cells lies in their ability to provide electricity, heat, and even cooling simultaneously. The electricity generated can power various devices, from electric vehicles to household appliances. The heat produced can be utilized for space heating or industrial processes, offering a versatile and efficient energy solution. Moreover, the cooling effect is a byproduct of the water formation process, making hydrogen fuel cells a comprehensive energy management system.
In the context of hydrogen fuel cars, the process is particularly fascinating. Hydrogen fuel cells power electric vehicles, providing a clean and efficient alternative to traditional internal combustion engines. The cars store compressed hydrogen in specialized tanks, which is then supplied to the fuel cell stack. Here, the hydrogen undergoes the same electrochemical reaction, generating electricity to drive the vehicle's electric motor. This technology promises zero-emission transportation, reducing our reliance on fossil fuels and mitigating the environmental impact of the automotive industry.
4-Cycle Fuel in Cars: What You Need to Know
You may want to see also

Oxygen Requirement: Hydrogen fuel cells require oxygen for combustion, but not for the fuel cell reaction
The concept of hydrogen fuel cells and their interaction with oxygen is an intriguing aspect of automotive technology. While it is true that hydrogen fuel cells do require oxygen for their operation, the process is quite different from traditional combustion engines.
In the context of hydrogen fuel cells, oxygen is indeed necessary, but not for the combustion process as we commonly understand it. Hydrogen fuel cells operate through a unique electrochemical reaction, where hydrogen gas (H₂) and oxygen (O₂) are combined to produce electricity, water, and heat. This reaction is highly efficient and produces no harmful emissions, making it an environmentally friendly alternative to conventional internal combustion engines.
The process begins when hydrogen gas is fed into the fuel cell, where it undergoes a reduction reaction at the anode. This reaction splits the hydrogen atoms, releasing electrons and forming protons. These electrons are then utilized to generate an electric current, which powers the vehicle. Simultaneously, at the cathode, oxygen from the air is combined with the protons and electrons to form water molecules, completing the reaction.
Here's the key point: unlike traditional combustion, where oxygen is required for the burning of fuel, in hydrogen fuel cells, oxygen is essential for the overall electrochemical reaction but not for the combustion of hydrogen itself. The combustion of hydrogen occurs internally within the fuel cell, and it is the subsequent reaction with oxygen that produces the desired energy output.
This unique characteristic of hydrogen fuel cells allows them to operate efficiently even in the absence of oxygen, as the reaction primarily relies on the electrochemical process rather than combustion. This makes hydrogen fuel cell vehicles highly adaptable and suitable for various environments, including urban areas with limited oxygen availability.
Flex Fuel Flexibility: Unleaded Compatibility Explained
You may want to see also

Air Intake: Cars with hydrogen fuel cells need air intake systems to supply oxygen for combustion
The concept of hydrogen fuel cell vehicles has sparked interest in a cleaner and more sustainable alternative to traditional internal combustion engines. One of the key questions that arises is whether these vehicles require oxygen for operation. Unlike conventional gasoline or diesel engines, hydrogen fuel cell cars do not rely on combustion as their primary power source. Instead, they utilize a process called electrochemical reaction, where hydrogen gas reacts with oxygen from the air to produce electricity. This reaction is highly efficient and produces only water as a byproduct, making it an environmentally friendly power source.
However, the absence of combustion means that these vehicles still need a way to supply oxygen to facilitate the electrochemical reaction. This is where the air intake system comes into play. In a hydrogen fuel cell car, the air intake system is designed to draw in fresh air from the atmosphere and direct it to the fuel cell stack. The air intake must be carefully engineered to ensure it provides the necessary oxygen while also filtering out impurities like dust and pollutants, which could potentially damage the fuel cell components.
The air intake system typically consists of an air filter, which traps contaminants, and a bypass valve to control the airflow. When the engine is running, the air intake system continuously supplies oxygen-rich air to the fuel cell stack. This oxygen is then combined with hydrogen gas, which is supplied from the fuel tank, to generate electricity through the electrochemical reaction. The efficiency of this process is crucial to the overall performance and longevity of the hydrogen fuel cell vehicle.
It is important to note that the air intake system in a hydrogen fuel cell car is different from that of a conventional engine. While a traditional engine requires a large volume of air for combustion, a fuel cell only needs a steady supply of oxygen to sustain the electrochemical reaction. Therefore, the air intake system is designed to provide a controlled and precise flow of air, ensuring optimal performance and minimizing energy loss.
In summary, hydrogen fuel cell cars do require oxygen for their operation, but it is not through combustion. Instead, they rely on an efficient air intake system to supply oxygen for the electrochemical reaction. This system plays a vital role in the overall functionality and environmental benefits of hydrogen fuel cell vehicles, contributing to a cleaner and more sustainable future for transportation.
Uncovering the Mystery: Do Cars Have a Hidden Fuel Reserve?
You may want to see also

Storage and Delivery: Oxygen storage and delivery systems are crucial for hydrogen fuel cell vehicles
The efficient storage and delivery of oxygen is a critical aspect of hydrogen fuel cell vehicles, ensuring their optimal performance and widespread adoption. Unlike traditional internal combustion engines, hydrogen fuel cells require a continuous supply of oxygen to facilitate the electrochemical reaction that produces electricity. This process involves the reduction of oxygen and the reaction with hydrogen to form water, thus generating electrical energy. Therefore, an efficient oxygen storage and delivery system is essential to support the operation of these vehicles.
One of the primary challenges in hydrogen fuel cell vehicles is the storage of oxygen. Unlike hydrogen, which can be stored in high-pressure tanks or through cryogenic methods, oxygen storage is more complex. The most common approach is to use compressed oxygen tanks, similar to those found in scuba diving equipment. These tanks store oxygen at high pressures, typically around 200-300 bar, to ensure a sufficient supply for the fuel cell stack. However, the high pressure and potential hazards associated with compressed gases require careful design and engineering to ensure safety and efficiency.
Oxygen storage tanks must be designed to withstand the extreme conditions of high pressure and temperature. Materials such as aluminum alloys or composite materials are often used to construct these tanks, providing strength and corrosion resistance. Additionally, safety mechanisms, such as pressure relief valves and overpressure protection systems, are implemented to mitigate potential risks. The size and weight of the oxygen storage system also need to be optimized to fit within the vehicle's structure while minimizing impact on overall performance and range.
In addition to storage, the delivery of oxygen to the fuel cell stack is another critical component. Oxygen must be supplied at a precise rate and pressure to ensure efficient operation. This is typically achieved through a network of pipelines or conduits that connect the storage tank to the fuel cell stack. The design of this delivery system must consider factors such as flow rate, pressure regulation, and the potential for leaks or contamination, as oxygen purity is essential for optimal performance.
Furthermore, the development of advanced oxygen delivery systems has led to innovations in the field. One such example is the use of ceramic membranes, which can selectively transport oxygen while blocking other gases. These membranes can be integrated into the fuel cell stack, providing a continuous and controlled supply of oxygen directly to the reaction sites. This technology offers improved efficiency, reduced weight, and enhanced safety compared to traditional compressed oxygen storage methods.
In summary, the storage and delivery of oxygen are vital components of hydrogen fuel cell vehicles. Efficient oxygen storage systems, often utilizing compressed tanks or advanced materials, ensure a reliable supply of this essential reactant. Meanwhile, sophisticated delivery systems, including pipelines or ceramic membranes, facilitate the precise delivery of oxygen to the fuel cell stack. These advancements in oxygen storage and delivery technology are key enablers for the widespread adoption of hydrogen fuel cell vehicles, contributing to their performance, safety, and overall viability as a clean energy alternative.
Are Flex Fuel Cars Reliable? Unlocking Robustness and Durability
You may want to see also

Environmental Impact: Hydrogen fuel cars produce water vapor and no harmful emissions, reducing air pollution
The environmental benefits of hydrogen fuel cell vehicles are significant, particularly in the context of reducing air pollution and combating climate change. One of the most notable advantages is that hydrogen fuel cars produce water vapor as their primary byproduct, rather than harmful emissions like carbon dioxide or nitrogen oxides. This is a stark contrast to traditional internal combustion engines, which release a multitude of pollutants into the atmosphere.
When hydrogen fuel cells generate electricity, they undergo a chemical reaction that combines hydrogen and oxygen, resulting in the formation of water. This process is highly efficient and produces no toxic gases or particulate matter, making it an environmentally friendly alternative to conventional combustion engines. The absence of harmful emissions means that hydrogen fuel cars contribute to cleaner air, especially in densely populated urban areas where pollution levels are often high.
The environmental impact of hydrogen fuel cell technology extends beyond the reduction of local air pollution. The production and use of hydrogen can be derived from renewable energy sources, such as wind or solar power, which further enhances its sustainability. By utilizing clean energy to generate hydrogen, the entire lifecycle of the fuel becomes environmentally friendly, from production to consumption.
Moreover, the widespread adoption of hydrogen fuel cell vehicles can play a crucial role in mitigating climate change. The transportation sector is a significant contributor to global greenhouse gas emissions, and transitioning to electric vehicles powered by hydrogen can substantially lower carbon footprints. As hydrogen fuel cells produce no direct emissions, their use can help reduce the overall carbon intensity of the transportation industry.
In summary, hydrogen fuel cars offer a promising solution to environmental concerns, particularly in the realm of air quality and climate change. Their ability to produce water vapor and lack of harmful emissions makes them a cleaner alternative to traditional vehicles. With the potential to utilize renewable energy sources for hydrogen production, these cars can contribute to a more sustainable and environmentally friendly future, paving the way for reduced air pollution and a healthier planet.
The Shift to Fuel Efficiency: What's the Appeal?
You may want to see also
Frequently asked questions
No, hydrogen fuel cell vehicles do not need oxygen to operate. These cars utilize a process called electrochemical conversion, where hydrogen gas is combined with oxygen from the air in a fuel cell, producing electricity through a chemical reaction. This reaction produces only water as a byproduct, making it an environmentally friendly and efficient power source.
In hydrogen fuel cell vehicles, the oxygen is supplied from the surrounding air. The fuel cell stack contains a catalyst that facilitates the reaction between hydrogen and oxygen. This catalyst splits the oxygen molecules (O₂) into two oxygen atoms, which then combine with hydrogen atoms (H₂) to form water (H₂O). This process is highly efficient and allows the car to generate electricity for propulsion.
No, there is no risk of explosion under normal operating conditions. The fuel cell system is designed to operate safely, and the reaction only occurs when both hydrogen and oxygen are present in the correct proportions. Additionally, the fuel cells are designed to prevent the buildup of excessive pressure, ensuring a safe and controlled environment. However, it's important to note that proper ventilation and maintenance are crucial to ensure the safe operation of hydrogen fuel cell vehicles.







